On August 15, the US Food and Drug Administration (FDA) approved a new method of testing for Covid-19 — processing saliva samples — and termed it “ground-breaking”.
Israel’s Center for Geographic Medicine and Tropical Diseases, too, has developed a saliva test that it claims will detect the novel coronavirus “in less than a second”, Reuters reported. That test, however, is still is in the process of getting regulatory approval.
In the first category of tests, nose and throat swabs are used as the sampling technique . After the swab is removed, the sample is placed in a viral transport media and is preserved for analysis. Researchers have developed the saliva test as a low-cost alternative; the suspected patient has only to spit into a sterile tube and the sample is then sent it to the laboratory.
In the last few months, the US FDA has authorised at least five diagnostic tests that use saliva samples. The first was on May 8 — emergency-use authorisation to Rutgers Clinical Genomics Laboratory’s molecular test, commercially manufactured by Spectrum Solution. This was after results showed the device could be used for collection, transportation, and storage, following home collection of saliva.
On August 15, the FDA issued emergency-use authorisation to Yale School of Public Health’s diagnostic kit, called SalivaDirect. “This is the fifth test that the USFDA has authorised that uses saliva as a sample for testing,” it said.

Don’t miss from Explained | What the serosurvey results in India imply
Story continues below this ad
What are the advantages of a saliva test?
First, as noted in a yet-to-be-peer-reviewed study by Yale researchers, saliva “does not require a certified swab and collection receptacle and does not necessarily have to be obtained by a skilled healthcare provider, both of which increase diagnostic-associated costs”. Therefore, cost is the biggest advantage.
Second is accuracy: A saliva test is reported to be prone to less error than has been reported in nasal swab collection, which sometimes give false negatives.
Third, the test is less invasive than a procedure that goes to the back of the nose to collect the swab. It is also safer for healthcare workers who collect the swab. Inserting the swab into the back of the nose often results in sneezing and coughing. Spectrum Solution claims that use of saliva kits delivers an over 90% reduction in PPE usage compared to current swab collections for Covid-19 testing.
Fourth, it is a convenient option that does not require travelling to a hospital or lab.

Story continues below this ad
📣 Express Explained is now on Telegram. Click here to join our channel (@ieexplained) and stay updated with the latest
Apart from sampling methods, how is the saliva test different from RT-PCR?
Like RT-PCR, a saliva test too converts the virus RNA into DNA, then amplifies the DNA to detect presence of the virus. What makes the SalivaDirect kit unique is that the Yale researchers have done away with a separate step or specialised equipment to extract the virus RNA.
“Our approach can be broadly implemented as it does not require saliva collection tubes containing preservatives and does not require specialized reagents or equipment for nucleic acid extraction,” the Yale study states.

What are the limitations of the saliva test?
In the case of the kit developed by Rutgers, the specimens must be transported and stored at ambient temperature and tested within 48 hours of collection.
Story continues below this ad
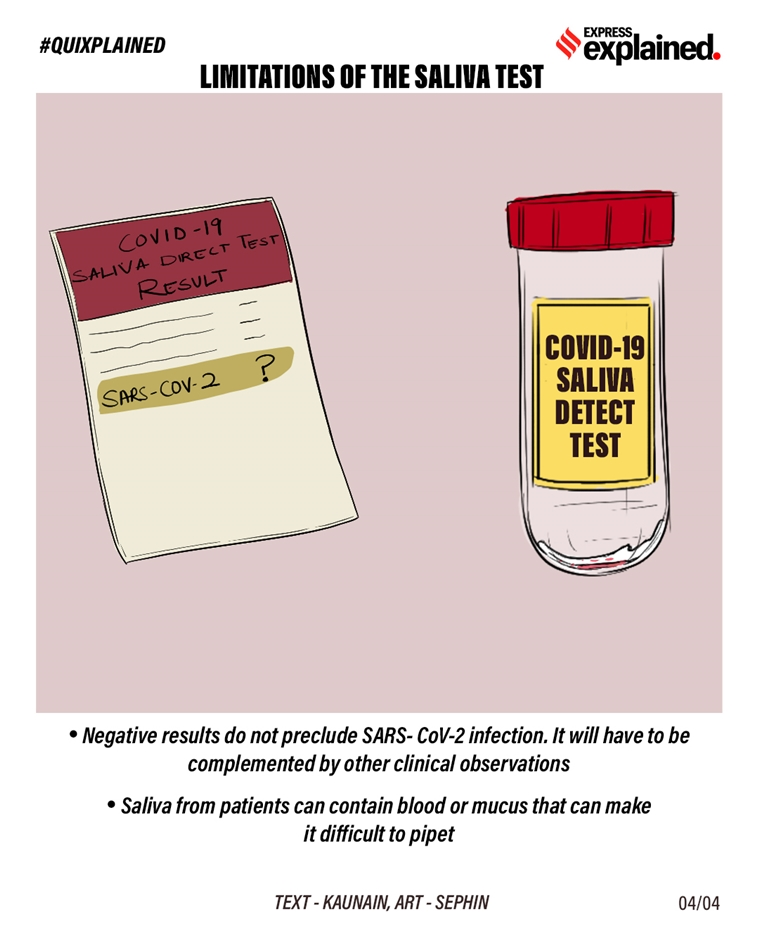
Again, the possibility of false negatives remains. As noted by the US FDA, SARS-CoV-2 nucleic acid is generally detectable in saliva specimens during the acute phase of infection. “Negative results do not preclude SARS- CoV-2 infection and should not be used as the sole basis for patient management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information. Negative results for SARS-CoV-2 RNA from saliva should be confirmed by testing of an alternative specimen type if clinically indicated,” the FDA states.
The Yale researchers have also flagged the fact that saliva from patients can contain blood or mucus, which can interfere with testing and results.

Where are saliva tests being used?
Yale’s saliva test, even before it got emergency approval, was tested on a group of National Basketball Association players and support staff. “ SalivaDirect is being further validated as a test for asymptomatic individuals through a program that tests players and staff from the NBA,” Yale University said.
The Rutgers test too has been used by NBA teams and other sportspersons.