There is no fixed period but the process can typically run into decades — and still yield no results. For instance, after three decades of research, the vaccine for HIV is still in phase III of clinical trials. One of the fastest developed vaccines is the one used for mumps, which received approval in four years after trials began in 1963.

In March, Antony Fauci, Director of the US National Institute of Allergy and Infectious Diseases, told the Senate Committee: “We would not have a vaccine that would even be ready to start to deploy for a year to year and a half. That is the time frame.” However, even this ambitious target can only be achieved when regulators give emergency authorisation, and not through the usual process that is followed for approvals.
In general, a vaccine is developed and tested over a number of stages. For Covid-19, this has been fast-tracked in various ways.
 Top Covid-19 vaccine candidates
Top Covid-19 vaccine candidates
Stage I: R&D
This typically takes two to four years. For Covid-19, this stage has been progressing fast for two reasons. First, a large number of candidates are based on the virus’s genetic code instead of its protein, and Chinese researchers globally shared the genetic sequence in January itself. The second reason is technology. For instance, Moderna is using m-RNA technology, which involves injecting genetic instructions to human cells for creating proteins to fight the virus. However, it is still unproven technology.
Stage II: Pre-clinical
This is when scientists test the vaccine on cell cultures and animals. They first inactivate the virus, pull out parts of the genetic sequence, and test if it triggers an immune response. More critically, they check if vaccine candidate continues to harm the cell. If there is no immune response and if the candidate harms the animals, the researchers return to stage 1. This stage can take two to three years.
Story continues below this ad
For Covid-19, this stage is being shortened by performing various sub-stages simultaneously. However, most vaccine candidates are still in the pre-clinical stage.
Stage III: Clinical trials
On the basis of data submitted from the pre-clinical phase, regulators allow testing in humans. Very few candidates enter this stage. This phase consists of three phases and usually takes more than 90 months.
PHASE I: The vaccine is given to a small group of people — this takes about three months — and scientists measure antibodies in their blood.
PHASE II: If found safe, it moves to the next phase (6-8 months). The vaccine is given to several hundred people. Three aspects are assessed: reactogenicity (ability to produce common, adverse reactions), immunogenicity (ability to provoke an immune response) and safety. There is also a control to compare how the vaccine works in different variables.
Story continues below this ad
This stage has been shortened in Covid-19 vaccine development. Moderna took just 63 days to reach clinical trials. The Oxford Vaccine Group researchers, which began phase I trials of Astra Zeneca’s vaccine in April, has now entered phase III.
PHASE III: Thousands of people are enrolled. This takes 6-8 months. This assesses how the vaccine works in larger populations.
📣 Express Explained is now on Telegram. Click here to join our channel (@ieexplained) and stay updated with the latest
Stage IV: Regulatory review
The manufacturer submits the data to receive a licence. In the US, approval typically comes after 10 months. However, this is fast-tracked during emergencies. The regulators allow a rolling review: the vaccine candidate submits sections of the application for review as and when they are completed.
Stage V: Manufacturing
Story continues below this ad
This requires immense resources — funds running into millions of dollars, infrastructure, raw material, and scientific expertise. Pharma giants like Pfizer, Johnson & Johnson, Merck and Astra Zeneca, all trying to develop a vaccine, will have a clear advantage to scale up manufacturing if their product is found successful.
Stage VI: Quality control
The safety of the vaccine is monitored by both the regulator and the manufacturer.

 There is no fixed period but the process can typically run into decades — and still yield no results. (AP Photo: LM Otero, File)
There is no fixed period but the process can typically run into decades — and still yield no results. (AP Photo: LM Otero, File)
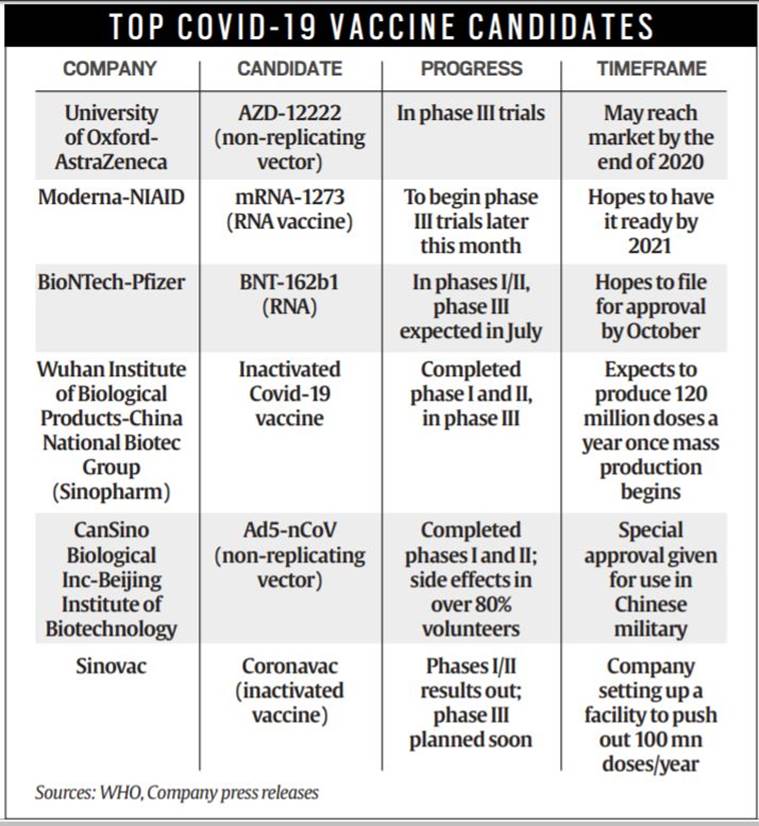 Top Covid-19 vaccine candidates
Top Covid-19 vaccine candidates




































