Have you seen the crystal structure of bleach?
When asked if decoding the structure can help build a better bleach, Frišcic told C&EN magazine: “Probably not...It’s not a breakthrough, but it’s really cute.”
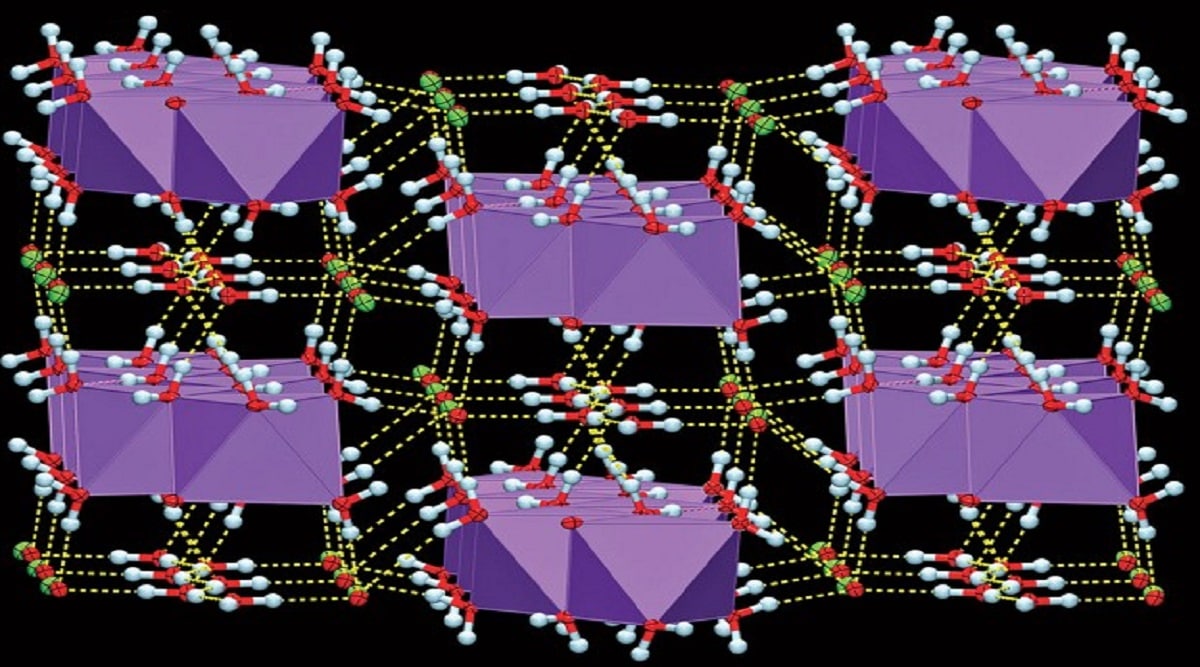 A crystal of sodium hypochlorite contains alternating layers of hydrated Na+ ions (purple octahedral) and chains of water molecules (oxygen = red; hydrogen = white) flanked by ClO– ions (chlorine = green), held together by hydrogen bonds (yellow dashes).
(Angew. Chem., Int. Ed. via C&EN)
A crystal of sodium hypochlorite contains alternating layers of hydrated Na+ ions (purple octahedral) and chains of water molecules (oxygen = red; hydrogen = white) flanked by ClO– ions (chlorine = green), held together by hydrogen bonds (yellow dashes).
(Angew. Chem., Int. Ed. via C&EN)For over 200 years, humans have used bleach or sodium hypochlorite(NaOCl) as a disinfectant. Available in powder and solution form, it is a common household chemical and has applications in paper and textile industry. Despite this long history and even longer list of uses, its crystal structure has just been determined.
Last month, a paper published in the journal Angewandte Chemie provided the first X-ray single crystal structure of hydrated sodium hypochlorite.
The structure of bleach! Long-missing single crystal structures of hypochlorite and hypobromite salts now reported by @filip_to Joe Marrett @TH_Borchers @hatemtiti85 Chris Barrett @mcgillu @McGillChemistry @angew_chem https://t.co/5EnK6d76Jv
— Tomislav Friscic (@TomislavFriscic) July 22, 2021
“I think it’s one of those things that was hiding in plain sight, that somehow slipped through the cracks,” said McGill University’s Tomislav Frišcic, who led the work, to Chemical & Engineering News(C&EN) magazine.
Since solid sodium hypochlorite liquefies at room temperature, the team did X-ray diffraction studies at –100 °C. They saw alternating layers of hydrated sodium (Na+) and hypochlorite (ClO–) ions and chains of water molecules, “There is a ton of hydrogen bonds in the structure, which hold everything together,” Frišcic said.
They noticed that sodium hypobromite (NaOBr) also had a similar structure.
When asked if decoding the structure can help build a better bleach, Frišcic told C&EN magazine: “Probably not…It’s not a breakthrough, but it’s really cute.”
Researchers have uncovered the crystal structure of bleach using x-ray crystallography for the first time since its original synthesis in the 18th century https://t.co/XoLaXJeSnb pic.twitter.com/cc5WJZ8bn8
— Chemistry World (@ChemistryWorld) August 17, 2021
Christine Beavers, an x-ray crystallographer at the UK’s Diamond Light Source, told chemistryworld.com that hypohalites are “the kind of things you learn in first year chemistry, I thought they were all pretty done and dusted”. She added that the procedure used in the study was “very standard crystallography, the most difficult thing was that the crystals seem to dissolve in themselves”.







