© The Indian Express Pvt Ltd
Latest Comment
Post Comment
Read Comments
 Oxford's vaccine for Covid-19 is AZD1222.
Oxford's vaccine for Covid-19 is AZD1222.Late last month, Oxford and AstraZeneca said its vaccine candidate for Covid-19, AZD1222, could have an efficacy up to 90 per cent — when administered in a half-dose followed by a full dose a month later. What they did not disclose initially was that these findings were the result of a mistake. The nearly 3,000 participants in the UK on whom this result was based were never supposed to be given a lower dose in the first place.
The revelation added to doubts on how vaccine clinical trials during the pandemic have been handled. Here’s a look at why it matters, and what needs to be done now:
 Oxford vaccine error has raised questions about the way clinical trials have been handled during the pandemic.
Oxford vaccine error has raised questions about the way clinical trials have been handled during the pandemic.
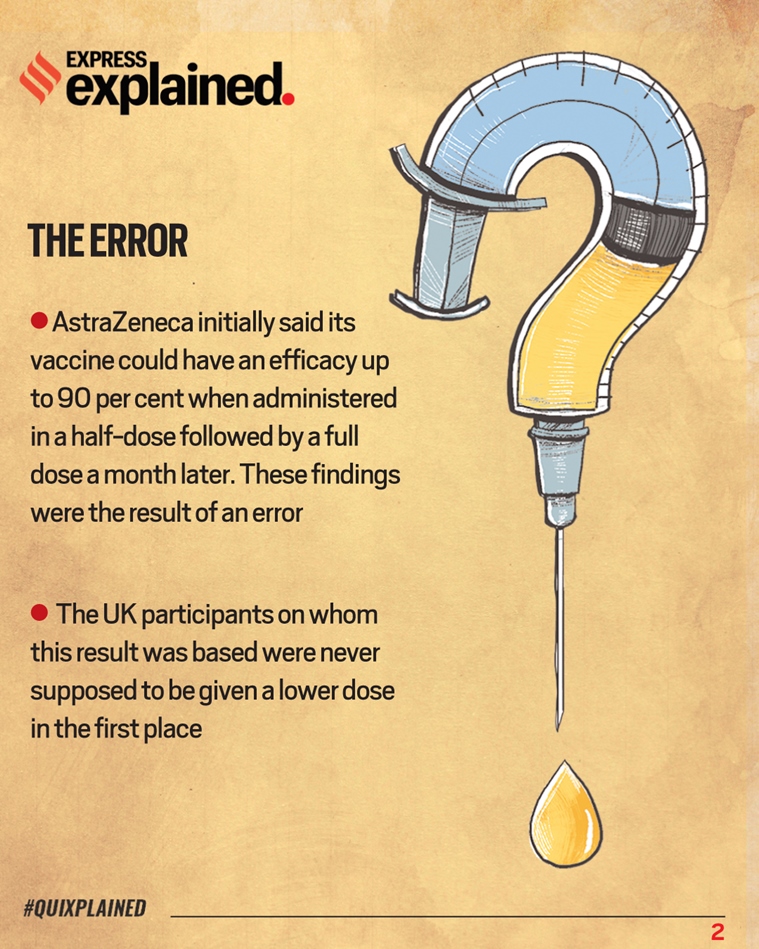 What was Oxford’s vaccine error?
What was Oxford’s vaccine error?
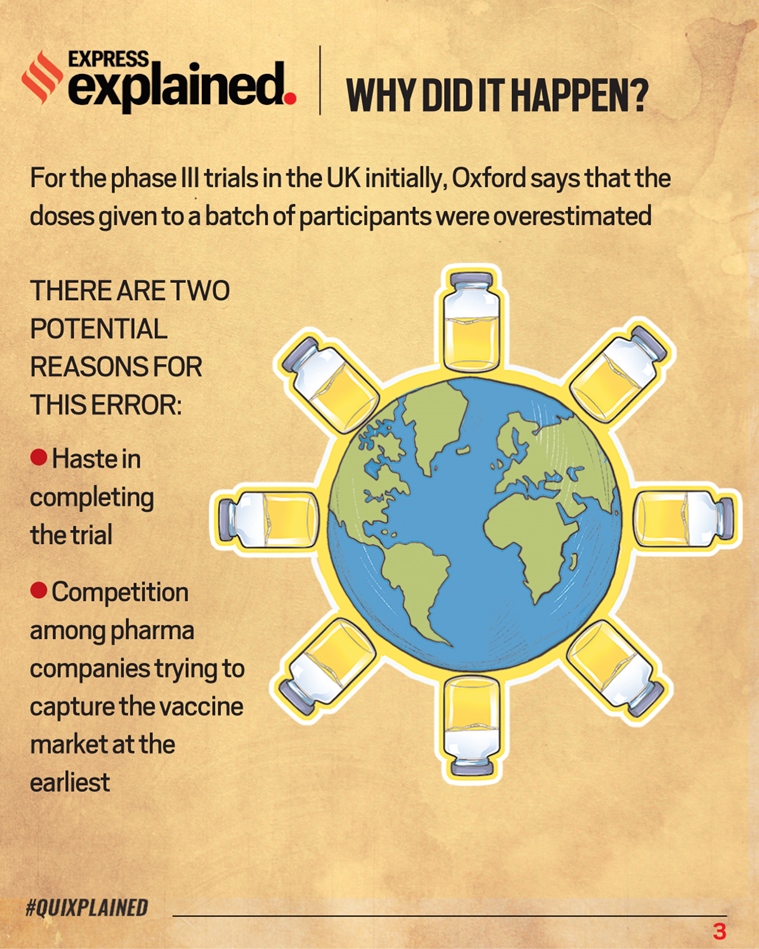 Why the Oxford vaccine error happen?
Why the Oxford vaccine error happen?
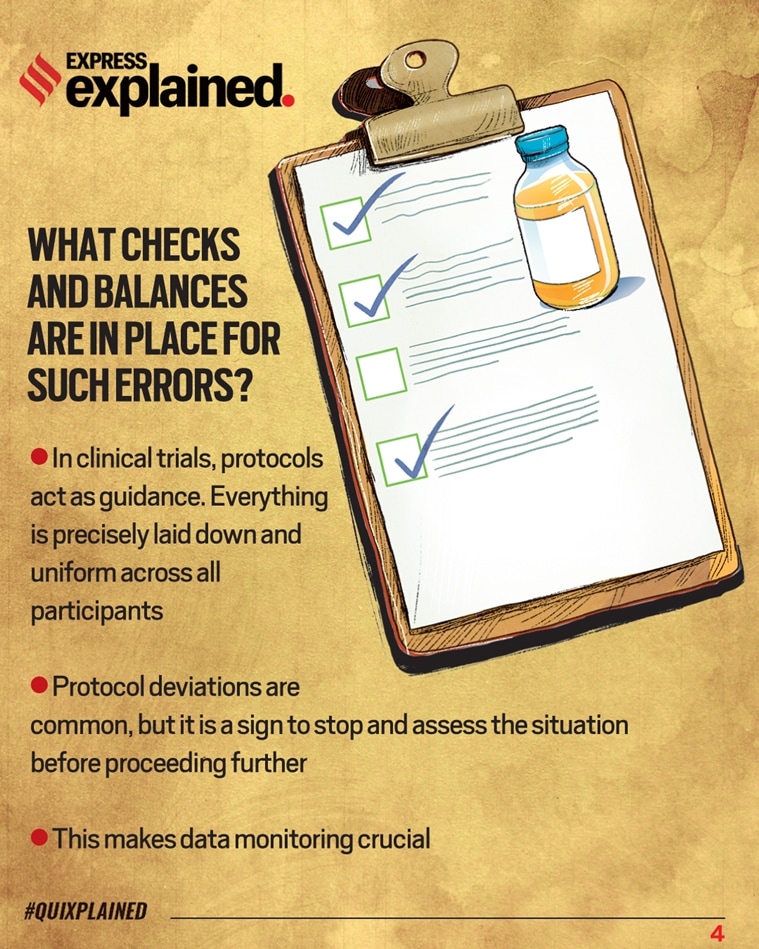 Oxford vaccine error: What checks and balances are in place for such errors?
Oxford vaccine error: What checks and balances are in place for such errors?
 Does Oxford’s vaccine error raise questions for India’s Covishield?
Does Oxford’s vaccine error raise questions for India’s Covishield?
Don’t miss from Quixplained | How a vaccine travels from factory to syringe
x