Bharat Biotech
BHARAT BIOTECH NEWS
Bharat Biotech launches oral cholera vaccine, gets DCGI nod to manufacture from Hyderabad plant
August 27, 2024 8:14 pm
BBIL has established large-scale manufacturing facilities in Hyderabad and Bhubaneswar with a capacity to produce up to 200 million doses of Hillchol, said Bharat Biotech Executive Chairman Krishna Ella at a press conference.
Over 30% Covaxin takers suffered from health issues after 1 year, claims BHU study
May 16, 2024 8:12 pm
Published in the journal Springer Nature, the study comes in the wake of UK pharmaceutical giant AstraZeneca admitting its Covid vaccine can cause rare side-effects of blood clotting and lowering of platelet count in UK court.
Bharat Biotech teams up with Serum-owned Bilthoven Biologicals to procure oral polio vaccines
April 02, 2024 2:28 pm
Through this collaboration, Bharat Biotech and Bilthoven Biologicals will jointly obtain regulatory approvals and licences required to commercially manufacture OPVs in India for global supplies.
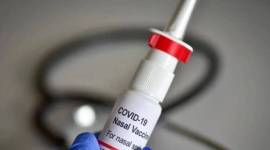
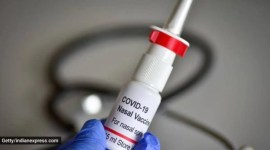
Intranasal vaccine launched; symbol of innovation: Govt
January 27, 2023 7:38 am
Clinical trials were conducted to evaluate iNCOVACC as a primary dose schedule and as a heterologous booster dose for subjects who have previously received two doses of either Covishield or Covaxin.
Covid-19: Bharat Biotech's intranasal vaccine to cost Rs 800 for private, Rs 325 for government supplies
December 28, 2022 9:31 am
iNCOVACC is the world's first intranasal vaccine for Covid to receive approval for the primary two-dose schedule, and as a heterologous booster dose.
COVID-19 vaccine booster shot: Here’s all you need to know about Bharat Biotech’s intranasal vaccine
December 27, 2022 12:11 pm
It is a good option because not only does it form neutralising antibodies but mucosal IGA antibodies and a T-cell response as well. It helps to prevent infection, disease and transmission, say experts
Bharat Biotech's intranasal Covid vaccine approved by govt, to be available on CO-WIN
December 23, 2022 11:18 am
The approval for the vaccine comes amid a spurt in Covid cases in China and some other countries.
Bharat Biotech's nasal vaccine could be a game-changer in the battle against Covid-19
September 08, 2022 8:10 am
Vaccines such as iNCOVACC can prevent mild cases of Covid and break the line of transmission of the disease
Nasal vaccine gets emergency use nod, how will it help combat Covid-19?
January 26, 2023 4:43 pm
Intranasal vaccines help overcome potential difficulties with mass vaccination and reduce the cost by doing away with the need for needles and syringes. But how do such vaccines work? We explain.
Bharat Biotech's intranasal Covid vaccine gets DCGI nod for restricted emergency use
September 07, 2022 6:18 am
Called iNCOVACC and manufactured by Bharat Biotech, the company behind Covaxin, the new vaccine has been approved for primary immunisation — it can be administered only to the unimmunised.
Best of Express