Latest research on fighting alzheimer’s disease: More Trials And Some Error
Data from continued drug trials by Eli Lilly released this week show it might be possible, if the drug is taken early, to slow Alzheimer’s in patients with a mild form of the disease. However, a different set of data, presented by Biogen, failed to show statistically significant benefits for patients on a closely regulated dose. The bottom line: A final assessment will take more time and tests, even though scientists think their line of attack on the disease is the correct one
THE ENEMY: PLAQUES
The drugs aim to block the formation of a protein called beta amyloid, which is believed to cause the toxic brain plaques that are considered a hallmark of Alzheimer’s. Beta amyloid is believed to build up in the brain for 10 or 15 years and steadily kill neurons before Alzheimer’s symptoms appear.
Beta amyloid comes from a larger protein found in the fatty membrane surrounding nerve cells. Beta amyloid is chemically “sticky”, and when protein pieces clump together, they gradually build up into plaques.
While drugs aimed at reducing the plaques have not shown definitive success in stemming decline in cognition, the “amyloid hypothesis” remains the focus of Alzheimer’s research.

HOPE: SOLANEZUMAB
Eli Lilly reported in 2012 that its drug, solanezumab, had not worked overall in two large 18-month trials, but had shown signs of efficacy in some patients in early stages of the disease. While it began a third big trial restricted to this particular subset of patients, it also extended the older trials, in which all participants — including those who had thus far received a placebo — were given solanezumab for another two years.
This meant that patients originally in the placebo group started the drug 18 months later than those who had been receiving it all along. The intention of the “delayed start” study was this: if solanezumab was indeed slowing mental deterioration, patients who were new to the drug would not be able to catch up in cognitive ability with patients who had been started on it before them.
On Wednesday, Lilly reported results that showed that the late starters had, in fact, failed to catch up with patients who had been on the drug for longer. The gap in cognition, however, remained consistent, suggesting that the drug was continuing to work on all patients.
The new findings, however, don’t prove that Lilly’s solanezumab really works; the larger study currently under way won’t end until late in 2016.
SETBACK: ADUCANUMAB
In March, Biogen reported data on patients who had taken its drug, aducanumab, in doses of 1, 3 and 10 mg per kilogram of the patient’s weight for about a year.
The early-stage results were encouraging — a sharply slowed decline in cognition compared to a placebo. But the most effective dose, the highest one, had a high rate of a side effect, which was localised swelling in the brain.
The new data presented on Wednesday was from 30 patients who got the medium dose of 6 mg per kg. Ahead of the results, it was hoped that the medium dose would show stronger efficacy than the two lower doses reported in March, and fewer side effects than the highest dose.
It largely failed to deliver on those expectations, and showed no clear path ahead immediately in the study.
The trials carried out a Mini Mental State Examination, or MMSE, and a Clinical Dementia Rating Sum of Boxes, or CDR-SB — two key tests that evaluate everyday mental skills and recall. On MMSE, the 6 mg dose did worse than the 3 mg test, raising questions over dosage-based targeting of the disease. On CDR-SB, it failed to distinguish itself to any significant degree. Safety data, involving the ARIA (amyloid-related imaging abnormalities) side effect, also did not improve.
Biogen said it was “enthusiastic about phase 3 [of trials], and [were] doing [their] very best to start enrollment… as soon as possible”.
RE-TRIAL: GANTENERUMAB
Roche said on Wednesday that it would revive research on two Alzheimer’s drugs that suffered setbacks in tests last year. These two drugs, gantenerumab and crenezumab, work in the same way as their rivals from Eli Lilly and Biogen — by targeting protein plaques in the brains of Alzheimer’s patients.
A spokesman said crenezumab would now move into late-stage phase III development, and gantenerumab would be put through fresh clinical trials using higher doses. Data presented at the Alzheimer’s Association International Conference in Washington on Wednesday suggested that gantenerumab was clearing beta-amyloid plaques from the brain, but that the dose was too low.
DEMENTIA IS
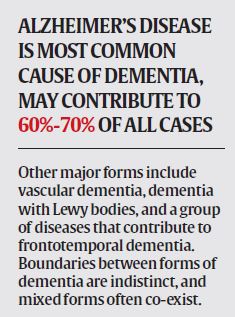
# Deterioration in memory, thinking, behaviour and ability to perform everyday activities
# Caused by a variety of diseases and injuries that affect the brain, such as Alzheimer’s disease or stroke. It is seen mainly in older people, but it is not a normal part of ageing
# Seen in 47.5 mn people worldwide, 58% of them in low- and middle-income countries. It occurs in 5 to 8 people per 100 in the age group of 60 and above. 7.7 million new patients are added every year worldwide, and global incidence is projected to hit 75.6 million by 2030, and 135.5 million by 2050
# Estimated to cost US $ 604 billion, or about 1% of the world’s GDP (global societal costs estimated in 2010)
# Believed to be preventable, to some extent, by focusing on the same risk factors as exist for vascular disease, i.e., diabetes, midlife hypertension, midlife obesity, smoking and physical inactivity
SYMPTOMS
# Dementia affects each person differently. Symptoms are seen in three stages.
# EARLY: Onset gradual; common symptoms include forgetfulness, losing track of time, becoming lost in familiar places
# MIDDLE: Signs and symptoms become clearer, more restricting: becoming forgetful of recent events, people’s names; becoming lost at home; having difficulty communicating; needing help with personal care; behaviour changes including wandering and repeated questioning
# LATE: Near total dependence; serious memory disturbances: becoming unaware of time and place; difficulty recognizing relatives, friends; increasing need for assisted self-care; difficulty walking; behaviour changes including aggression
TREATMENT GOALS
# No cure is available. It helps to:
# Diagnose as early as possible
# Optimise physical health, cognition, activity and well-being
# Identify and treat accompanying physical illness
# Detect and treat behavioural and psychological symptoms
(Adapted from Agency reports, THE NYT, and Alzheimer’s Association WEB SITE)
- 01
- 02
- 03
- 04
- 05






































