Novo Nordisk, the Danish pharmaceutical company that manufactures the popular weight-loss drug Wegovy and diabetes medication Ozempic, has asked authorities in the United States to stop the compounding of these products saying that this could pose safety risks.
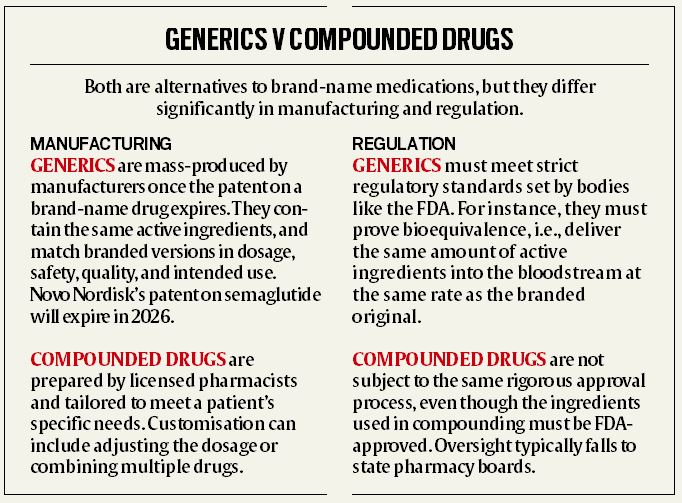
US Food and Drug Administration (FDA) regulations allow human drug compounding — in which licensed pharmacists or physicians can combine, mix, or adjust the ingredients of a medication — to meet patient needs when popular branded formulations are in short supply.

Wegovy and Ozempic, both of which contain semaglutide, have been in heavy demand for months, and many compounding pharmacies in the US have created their own versions to cope with this.
Previously, American drugmaker Eli Lilly had sought to stop compounded versions of its diabetes and obesity medications Mounjaro and Zepbound. The FDA is yet to make a decision on the applications.
Concerns over semaglutide
Over the past year, Novo Nordisk has filed at least 50 lawsuits against clinics and pharmacies that have produced compounded versions of its drugs. These copies could be dangerous, the company fears — Chief Financial Officer Karsten Munk Knudsen said last week that reports had linked at least 10 deaths and more than 100 hospitalisations to compounded versions of Wegovy and Ozempic.

On October 22, Novo Nordisk asked USFDA to put semaglutide on the Demonstrable Difficulties for Compounding (DDC) list, which restricts pharmacies from compounding a drug, especially when commercial versions are available.
The FDA considers a drug for the DDC list if factors such as its stability, dosage requirements, bioavailability, or necessary sterile handling make it difficult to create a safe and effective compounded version.
Story continues below this ad
In its application for DDC listing, Novo Nordisk has flagged four specific concerns to the FDA.
Complex formulation: The complex structure of semaglutide is hard to replicate accurately. The drug is manufactured using yeast and recombinant DNA technology, which gives it specific properties essential for its performance and safety. Replicating it synthetically can result in differences in purity, stability, and overall effectiveness.
The FDA-approved version of semaglutide includes specific components — such as the fatty acid — that determine how long it stays effective in the body, and synthetic versions may not perform similarly.
It has also flagged that compounded versions have led to reported adverse effects, which have been recorded in the FDA’s Adverse Event Reporting System (FAERS).
Story continues below this ad
Delivery mechanism: The delivery mechanism of a drug refers to the way it is sent and released into the body so that it works effectively.
According to the company, the FDA-approved semaglutide uses sophisticated delivery methods, the precision of which helps the drug work effectively and safely. Compounded versions often lack these advanced delivery mechanisms, which can lead to the drug not being released in the proper dose, and reduce its effectiveness and put patient safety at risk.
A safe and effective delivery mechanism is especially important because patients administer the drug themselves. The FDA-approved version of semaglutide, made by Novo Nordisk, comes in a single-use pen injector, which provides the exact dose, and includes clear instructions that ensure against patients accidentally taking too much of the drug. The compounded versions, on the other hand, often come in multi-dose vials or prefilled syringes, with inconsistent instructions that increase the risk of dosing errors.
Novo Nordisk has cited examples of patients who overdosed on compounded semaglutide. In one case a man accidentally took 10 times the dose, resulting in severe nausea and vomiting.
Story continues below this ad
Bioavailability: This refers to the degree to which the active ingredient in a drug reaches the bloodstream and becomes usable by the body.
The company has said that semaglutide has naturally low bioavailability — and a compounded version that is not absorbed properly may not produce the intended effects. Ineffective treatment of obesity and diabetes can result in heart attacks, strokes, nerve damage, kidney disease, and may even require amputations.
Compounded semaglutide may not meet the requirements for effective bioavailability, the company has told the FDA.
Contamination, stability risk: The company has underlined that compounding semaglutide requires specialised facilities and equipment, and that the risk of contamination is a major concern. Contamination with other ingredients can potentially occur if the equipment is not sanitised thoroughly.
Story continues below this ad
According to the company, in March 2022, the FDA found issues with sterility at a compounding pharmacy that had had five sterility failures with injectable drugs in one year, and had released products before confirming they were sterile. More than 15,000 injectable semaglutide units were recalled, the company said — and another recall had followed in August 2023 for the same reasons.
Novo Nordisk has also said that semaglutide is a temperature-sensitive drug and storing it at temperatures higher than 30 degrees Celsius can compromise its stability.
The company has flagged an instance of a pharmacy instructing patients to keep a sublingual (under-the-tongue) solution at room temperature, and another advising them to freeze semaglutide products.