An Expert Explains: ‘Coronavirus tests needed, but each country has priorities’
As community spread of the novel coronavirus in India looms, answers to some key questions over testing for the virus.
 Testing for coronavirus: Wearing a protective suit, a research and development company employee works on the production of coronavirus testing kits in Gebze, northwestern Turkey, Monday, March 23, 2020. (AP Photo: Emrah Gurel)
Testing for coronavirus: Wearing a protective suit, a research and development company employee works on the production of coronavirus testing kits in Gebze, northwestern Turkey, Monday, March 23, 2020. (AP Photo: Emrah Gurel)
As India begins to discover its first cases of community transmission of the novel coronavirus, the number of infections is expected to shoot up very rapidly. Two days ago, the government decided to test all patients with severe respiratory disease in hospitals across the country, expanding the net beyond only those with recent overseas travel and contact histories.
Several experts have argued that India’s testing has been inadequate, and that the virus may be spreading widely outside the selected group.
Dr Jaya Shrivastava, a molecular biologist of infectious diseases for over 20 years, works for Public Health England. She spoke to The Indian Express:
What would be an adequate number of tests for any country?
COVID-19 is a barely three-month-old disease, and there is not enough data to recommend an adequate number for testing. What we need to understand is that testing is not a treatment, nor is it sustainable to test every suspected case, let alone every individual. In most cases, we test in order to decide on clinical management of the case. We also test because we need to identify if the infections are occurring through travellers and migrants or whether community transmissions have started. Tests are also used to ascertain levels and transmission trends of the infections within a population in order to decide intervention strategies like self-isolation, social distancing and quarantine procedures. Outcomes of testing also directly feed into a nation’s response, health strategy, and governance policies. Collectively, they help to break the chain of transmission.
We have to realise that in an epidemic-like situation for a highly infectious disease, when transmission happens rapidly, it is extremely difficult, if not impossible, to get ahead of the curve simply by testing more and more people. However, we do need to test as many people as we can. This was stressed by the WHO Director-General, who said his key message was to “test, test, test or we will be fighting a fire blindfolded”.
Don’t miss from Explained | When to test, and whom
However, all countries have limits to their capacity to test. Diagnostic prowess, trained manpower, lab infrastructure, all are limited. Countries, therefore, have to define their priorities based on their local risk assessments. And that is what they seem to be doing. We should remember that what holds true for Singapore or South Korea might not hold true for India.
In the current situation, I find the advice from Professor Graham Medley (professor of infectious disease modelling at the London School of Hygiene & Tropical Medicine) the most pertinent: “Act like you are infected, not just avoiding infections.” People should act like they have already tested positive for COVID-19, and then follow all the instructions that an infected person is being asked to follow, to the maximum extent possible: isolation, social distancing, minimal contact with other people.
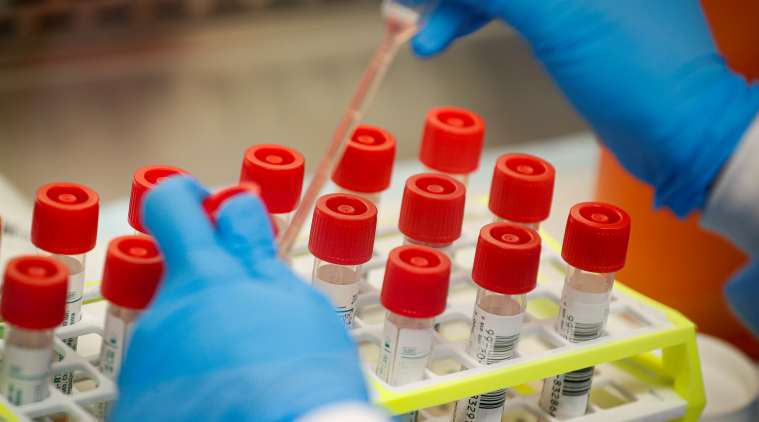 Testing for coronavirus: A technician prepares COVID-19 coronavirus patient samples for testing at a laboratory in New York’s Long Island. (AP Photo: John Minchillo)
Testing for coronavirus: A technician prepares COVID-19 coronavirus patient samples for testing at a laboratory in New York’s Long Island. (AP Photo: John Minchillo)
What exactly is a ‘testing kit’ for COVID-19? What is in it?
There is no composite ‘kit’ as of now. What we refer to as a “kit” is actually a bunch of chemicals and biochemicals that are required at different stages of testing, which include nucleic acid extraction and amplification. These chemicals and biochemicals usually do not come together, and have to be procured separately.
The presence of a virus is detected by looking for unique identifiers in a given sample. In the present case, we look for genetic material that we know are unique to this virus. The tests vary according to the particular genetic material that we are looking for. The WHO has published a list of several such tests in use in various countries. No one test is technically superior to another. Each of them is equally reliable. Countries are free to adopt any of these tests. The choice usually depends on factors like what works in the hands of a specific lab in terms of specificity, sensitivity, and logistics, including ease of availability of the chemicals required. The current molecular assays usually take between four and six hours to give the final result.
India has placed an import order for 1 million testing kits. What can be a realistic timeline for delivery?
There is no dearth of manufacturers who produce and supply the high-quality chemicals required in these tests, but they may not have the capacity to cater to the unprecedented and unexpected global demand volumes. I guess there would be a backlog of orders in most countries. For example, orders for these chemicals during ‘peace-time’ would materialise within 48 to 72 hours. Currently, however, it might take longer.
Also read | Can a person have coronavirus even if their respiratory samples test negative?
Can’t these chemicals be produced by Indian companies?
Indian companies already manufacture these chemicals. In fact, most research labs that perform molecular assays in India would have these chemicals; they are not uncommon at all. But the labs may not have them in the amounts they are currently required. Similarly, Indian companies that make these chemicals may be facing production issues. In my opinion, the bottleneck right now is of scale. Small manufacturers may not be in a position to supply such huge amounts.
The second issue is that of quality. With large and established players, quality is usually assured, but such quality assurances may not be readily available with suppliers that start production to address this new situation. Regulatory processes should be introduced to tackle this.
The third issue is that of cost. The bigger and established players can probably provide the chemicals for cheaper than small manufacturers can.
 Testing for coronavirus: Wearing a protective suit, a research and development company worker holds coronavirus testing kits in Gebze, northwestern Turkey, Monday, March 23, 2020. (AP Photo: Emrah Gurel)
Testing for coronavirus: Wearing a protective suit, a research and development company worker holds coronavirus testing kits in Gebze, northwestern Turkey, Monday, March 23, 2020. (AP Photo: Emrah Gurel)
How much do these tests cost?
The overall cost of the test would include the cost of materials that are required for the testing, as well as the cost incurred on lab infrastructure, technical expertise, and logistics. It would vary from country to country.
Many new tests are entering the market on a daily basis. These include molecular and serological assays; even artificial intelligence. These new tests will definitely drive down costs. However, the tests will need to be fit-for-purpose. Such analyses are already under way on a global level for all relevant new kits and assays.
Also read | Coronavirus testing in India, elsewhere
Has India delayed roping in private hospitals and labs for testing?
This is a novel disease, and we are all learning more and more with each passing day. Most countries began their COVID-19 incidence handling through their public health systems, and later drew in the private sector. In India, the initial testings were done by ICMR and their approved labs. ICMR also provided reagents, primers and probes, controls, testing protocol and guidance to its approved labs, possibly also testing material for the time being, training and software.
I should point out a very important factor regarding handling and processing of this virus in the laboratory. Sars-CoV-2 is currently categorised as a hazard group 3 (HG-3) organism that poses severe disease risk. As a result, certain stages of sample handling need to occur within appropriate containment facilities, which might not readily be available at all diagnostics labs. Also, while many private hospitals and laboratories in India may be able to meet the generic quality standards, their competency to perform COVID-19 specific assays would need to be approved and monitored on an ongoing basis.
Dr Jaya Shrivastava, a molecular biologist of infectious diseases for over 20 years, works for Public Health England. Views are personal.
Here’s a quick Coronavirus guide from Express Explained to keep you updated: Are smokers at high risk form coronavirus? | Can Vitamin-C prevent or cure coronavirus infection? | What exactly is community spread of coronavirus? | How long can the Covid-19 virus survive on a surface? | Amid the lockdown, what is allowed, what is prohibited?
- 01
- 02
- 03
- 04
- 05






































