Global Pharma Healthcare, a Chennai-headquartered company, whose eye drop has been linked to 55 adverse events including loss of vision and a death due to blood-stream infection, has been directed to halt production of all “ophthalmic preparations” following a joint inspection by the central and state drug control officers.
“The manufacturer was instructed to stop the manufacturing activities of all the products under the category of ophthalmic preparation till the completion of investigation,” said a report of the joint inspection carried out on Friday.

Global Pharma Healthcare manufactures at least 14 eye products, according to its website. Other than eye products, the company manufactures cardiovascular drugs, antibiotics, diuretics, painkillers along with several creams, ointments and syrups. The company manufactures drugs for over 30 countries, according to its website.
The investigation report states that the company had exported 24 batches of the product in question in two consignments. The product was manufactured in 2021 and 2022. Although the drug inspectors did not find any stock of the product at the company, they collected the control samples maintained by the company. Control samples are products from the same batches exported and maintained by companies as part of the quality control process.
Other than the control samples, the officials also collected samples of the raw material – Carboxy Methyl Cellulose Sodium – which was used to manufacture the product.
The investigation report states that the company carried out “media fill validation” biannually for which the records were verified. The media fill validation test is used to check microbial contamination of the manufacturing surfaces by exposing growth medium to the areas. It was also found that the company had undertaken yearly stability studies, in which the products are placed in controlled environments to check whether they meet the mentioned specifications. The investigation report said that a root cause analysis for the current complaint is underway at the company.
“The batch manufacturing records of all batches, purchase invoice of raw materials, COA (certificate of analysis) of raw materials, sales bill copies, media fill validation reports were obtained… for further investigation,” according to the joint inspection report, a copy of which is with ‘The Indian Express’.
Story continues below this ad
The company had initiated a voluntary recall of the artificial tear eye drop from the US market on Thursday after the US Food and Drug Administration (FDA) reposted its earlier alert. The very next day, a team of six officials – three from the Central drug control authority and three from the state drug controller – inspected the company’s manufacturing facility situated 40 kms south of Chennai.
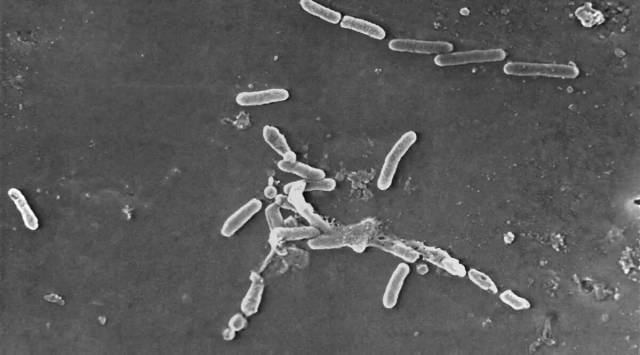
In its recall announcement, the company said the US Centre for Disease Control and Prevention alerted the FDA to initiate an investigation into a multi-state cluster of a bacterial infection pseudomonas aeruginosa resistant to the third-line antibiotic carbapenem. The over-the-counter eye drop was “distributed nationwide in the USA over the internet,” as per the company.
The incident comes on the heels of two cases of allegedly contaminated Indian manufactured syrups linked to the deaths of children in the Gambia and Uzbekistan. After similar inspections found that the companies in Haryana and Uttar Pradesh were not adhering to manufacturing practices, the facilities were shut down by the Indian regulator. A risk-based inspection of drug manufacturing companies was carried out thereafter.
In Uzbekistan, 18 children died of kidney failure allegedly after consuming two syrups manufactured by Marion Biotech. While in Gambia, the death of 70 children due to acute kidney injury has been linked to four syrups manufactured by Maiden Pharma. In both the cases, the syrups allegedly contained contaminants di-ethylene glycol and ethylene glycol.
Story continues below this ad
According to sources in the Union Health ministry, the eye drop in question is not sold in India.