Explained: Under scanner, what is Ranitidine, and should Indian users worry?
Ranitidine, popularly known through brand names like Aciloc, Zinetac, Rantac and Rantac-OD, R-Loc and Ranitin, is an over-the-counter, prescription antacid used in the treatment of acid reflux and peptic ulcer diseases.
 Ranitidine, popularly known through brand names like Aciloc, Zinetac, Rantac and Rantac-OD, R-Loc and Ranitin, is an over-the-counter, prescription antacid used in the treatment of acid reflux and peptic ulcer diseases.
Ranitidine, popularly known through brand names like Aciloc, Zinetac, Rantac and Rantac-OD, R-Loc and Ranitin, is an over-the-counter, prescription antacid used in the treatment of acid reflux and peptic ulcer diseases.
India’s drug regulator this week began looking into concerns of potential cancer-causing substances contaminating popular acidity drug ranitidine. The move came over a week after the US Food and Drug Administration flagged the issue to American patients, some companies have suspended sales of the product worldwide, and some other countries have ordered recalls of the product:
What is ranitidine, and how widespread is its use in India?
Ranitidine, popularly known through brand names like Aciloc, Zinetac, Rantac and Rantac-OD, R-Loc and Ranitin, is an over-the-counter, prescription antacid used in the treatment of acid reflux and peptic ulcer diseases. It is commonly used to relieve acid-related indigestion and heartburn by decreasing stomach acid production.
While other medicines like pantoprazole and omeprazole too treat these symptoms and are more commonly prescribed today, ranitidine is still widely used in India.
“Ranitidine is a much older medication, but it was always thought to be a very safe drug because it has less side effects than the other drugs that patients use nowadays to treat these symptoms,” said Dr S Chatterjee, senior consultant-internal medicine at Delhi’s Indraprastha Apollo Hospital.
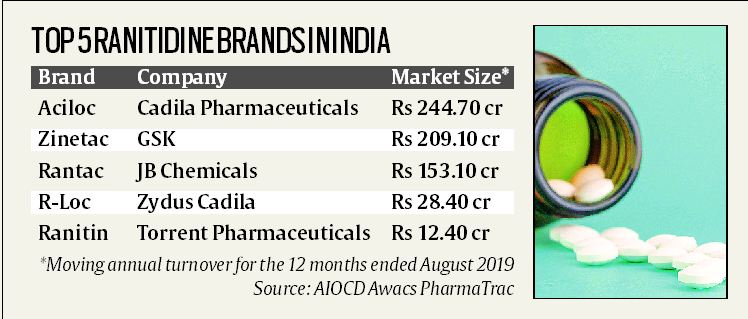
In the 12 months ended August 2019, the ranitidine molecule alone (excluding combinations it was part of) made nearly Rs 690 crore in sales, according to pharmaceutical market research firm AIOCD Awacs PharmaTrac.
What is the problem?
On September 13, the US FDA stated in a release that it had learned that some ranitidine medicines contained “low levels” of a substance called N-nitrosodimethylamine (NDMA). An environmental contaminant found in water and foods, NDMA has been classified by the International Agency for Research on Cancer as probably carcinogenic to humans, which means it has the potential to cause cancer.
This is the same impurity that the US FDA had investigated in blood pressure drugs valsartan and losartan over the last year.
How has India’s drug regulator responded?
India’s top drug regulator Monday wrote to state regulators asking them to direct ranitidine active pharmaceutical ingredient (API) manufacturers to “verify their products and take appropriate measures to ensure patient safety”.
Drugs Controller General of India (DCGI) V G Somani, in his letter, asked states to inform him of action taken in this matter “at the earliest”. So far, the DCGI has not called for any halting of supplies, which means the ranitidine brands marketed in the country can continue to be sold until further notice.
APIs are the ingredients that give a medicine its therapaeutic effect. According to industry sources, most of the world’s supply of the ranitidine API comes from two Indian firms — Saraca Laboratories and SMS Lifesciences.
Don’t miss from Explained: Soot found in placenta, is foetus at risk?
Should consumers be worried?
The DCGI has not clarified whether doctors and consumers in India should use ranitidine with caution, nor has the US FDA called for individuals to stop taking the drug at this time.
“Although NDMA may cause harm in large amounts, the levels the FDA is finding in ranitidine from preliminary tests barely exceed amounts you might expect to find in common foods,” stated the US FDA, adding it was evaluating whether these levels of the substance posed a risk to patients.
How have companies selling ranitidine in India responded?
At least two of the companies marketing top ranitidine brands here have decided to take precautionary measures like halting sales while investigations into their safety are in progress. This includes GSK, which publicly announced a voluntary recall of its Zinetac brand on Wednesday.
Torrent Pharmaceuticals, too, has “stopped the sales of this product” till it concludes a “detailed assessment” of its Ranitin. JB Chemicals, testing its Rantac and Rantac-OD, plans to take a final call on sales of products “once the results are out”, according to the company’s president, Pranabh Mody.
SMS is evaluating its ranitidine API to ensure it is “void of this NDMA impurity or are within permissible limits” and that it foresees “business as usual” until then.
According to GSK, the European Directorate for the Quality of Medicines (EDQM) has suspended Saraca Laboratories’ certificate of suitability for its ranitidine API “with immediate effect.” It is unclear what action Saraca is taking.
It is not clear at this stage either what Cadila Pharmaceuticals, which markets top-selling brand Aciloc in India, and Zydus Cadila, which markets ‘R-Loc’, are planning to do.
How have other countries responded?
While India and the US are still looking into the issue, regulators of around 15 countries are learnt to have called for recalls of ranitidine sold in their markets. These include Singapore, Canada, Italy, Denmark, Finland, Norway, Switzerland and Pakistan.
Singapore’s Health Sciences Authority has said the potential risk of nitrosamines like NDMA is associated with “long term exposure” and patients prescribed the drug for short term use “may continue with their medicine”.
- 01
- 02
- 03
- 04
- 05





































