Indian Prime Minister Narendra Modi and UK Prime Minister Keir Starmer signed the India-UK free trade agreement on Thursday, allowing wider access to Indian exports in the UK markets. Pharmaceuticals and medical devices will enjoy zero-duty access under this agreement.
With most pharmaceuticals and medical devices exported to the UK already being duty-free, industry experts say easing regulatory norms are likely to have a bigger impact in facilitating Indian exports to the UK.

What does the trade deal say about the drugs and medical devices sector?
Story continues below this ad
The zero-tariff provisions are likely to make Indian generic medicines more cost-effective in the UK market, as per the agreement. “India exports USD 23.31 billion globally and the UK imports nearly USD 30 billion, but Indian pharma accounts for under USD 1 billion, indicating significant headroom for growth,” the sectoral outcome summary states.
When it comes to medical devices, the agreement says that surgical instruments, diagnostic equipment, ECG machines, X-Ray systems among others will not attract any duty. “This will reduce costs for Indian med-tech companies and make their products more competitive in the UK market,” the agreement says.
The agreement adds that India has emerged as a cost-effective alternative to China, given that the UK is trying to move away from reliance on Chinese imports after Brexit and Covid-19. The agreement says: “Indian manufacturers are poised to emerge as a favoured, cost-effective alternative, especially with zero-duty pricing for medical devices.”
What do Indian manufacturers have to say?
When it comes to pharmaceuticals, the agreement is unlikely to lead to a sudden, sharp increase in Indian exports to the UK. An industry expert, who did not want to be named, said, “The pharmaceutical industry is likely to continue growing at its current trajectory — at around 10 per cent every year — because most of the drugs were already exempt from duty in the UK. What will help is easing of the regulatory framework, which will allow more Indian companies to enter the UK market and quickly.” Companies may end up spending thousands of dollars in getting the requisite approvals to sell in the UK.
Story continues below this ad
The same is true for medical devices as well. Rajiv Nath, forum coordinator of Association of Indian Medical Device Industry (AiMeD), said, “Medical devices imported from India by UK were duty-free previously as well, so tariff restrictions were not an issue. It was the time and cost of regulatory approvals that were a challenge.” He added that the industry had previously sought the recognition of approvals granted by the country’s apex drug regulator, CDSCO, or voluntary Quality Control of India certification for fast-tracking regulatory approvals in the UK.
Nath added that the lowering of the 7.5 per cent duty levied by India on most UK manufactured medical devices would hopefully happen in a phased manner.
 Pharmaceutical export
Pharmaceutical export
He added another caution: “There is a need for strict verification of the rules of origin of the medical devices in order to ensure that products manufactured by a third country are not routed through the UK for the tariff benefit. This is to ensure that India’s nascent medical device industry remains competitive.”
How many pharmaceutical products does the UK import from India?
Story continues below this ad
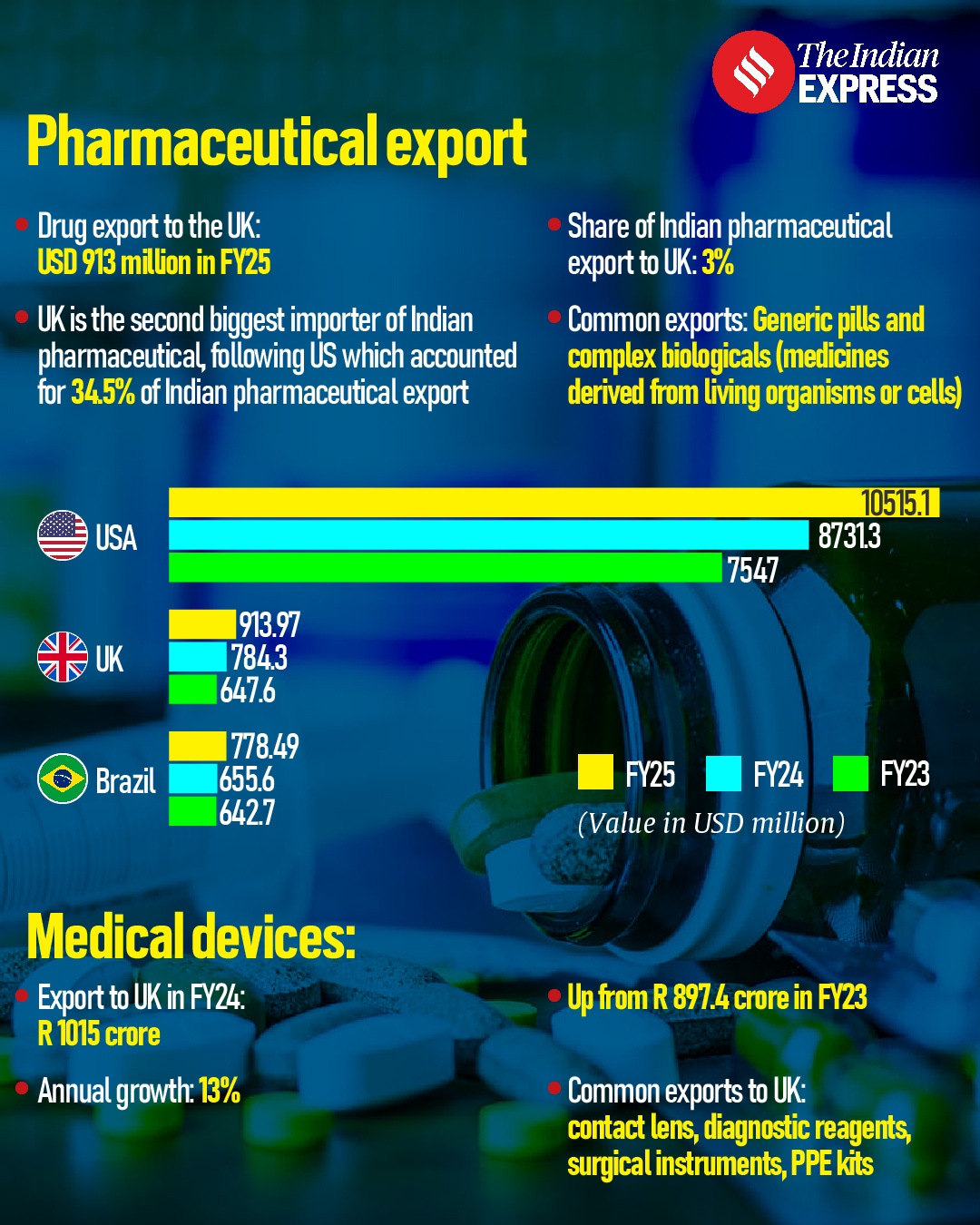
UK is the second largest importer of Indian pharmaceutical products, having purchased goods worth USD 913.97 million in FY2025. The country accounted for three per cent of all pharmaceutical exports from India. The largest importer of Indian pharmaceuticals continues to be the US, accounting for 34.5 per cent of the total exports, amounting to USD 10,515 million.
The most commonly exported products from India are generic versions of small-molecule pills as well as biologicals (medicines derived from living organisms or cells).
What’s the quantum of medical devices that the UK imports from India?
Export of Indian medical devices to the UK went up by 13 per cent in FY24 as compared to the previous year. These devices were worth Rs 1,015 crore, up from Rs 897.4 crore the previous year. The most common exports to the UK were contact lenses, diagnostic reagents, surgical instruments and PPE kits.


 Pharmaceutical export
Pharmaceutical export