‘First’ patient free of cancer: Indigenous CAR-T cell therapy brings treatment cost down from Rs 4 crore to Rs 40 lakh
Developed by ImmunoAct, IIT Bombay and Tata Memorial Hospital, the therapy has been administered to 15 patients in India. Three of them have successfully achieved cancer remission. Dr Gupta, the first commercial patient to be declared free of cancer, speaks of his experience exclusively to The Indian Express
 Dr (Col) V K Gupta has been declared cancer-free since he took the indigenously developed CAR-T cell therapy at Mumbai’s Tata Memorial Hospital. (Express photo by Gajendra Yadav)
Dr (Col) V K Gupta has been declared cancer-free since he took the indigenously developed CAR-T cell therapy at Mumbai’s Tata Memorial Hospital. (Express photo by Gajendra Yadav)A year ago, if someone had told Dr (Col) V K Gupta, 64, that he would not just be declared “free of cancer cells” — despite a failed bone marrow transplant in 2022 — but would be able to get back to work in 2024, he would have brushed it off as polite optimism.
Months after India’s drug regulator approved the commercial use of CAR-T cell therapy, a pioneering treatment that genetically reprogrammes a patient’s immune system to fight cancer, Gupta, a Delhi-based gastroenterologist, became one of the first patients to access the therapy by paying Rs 42 lakh — a treatment that costs approximately Rs 3-4 crore abroad. Doctors at the Tata Memorial Hospital, where he underwent the procedure, said he is “currently free of cancer cells”, the first commercial patient to achieve that status.
“While it’s premature to claim a lifelong cure, the patient is currently free of cancer cells,” said Dr Hasmukh Jain, hemato-oncologist and associate professor at the Advanced Centre for Treatment, Research and Education in Cancer (ACTREC), Tata Memorial Centre, who performed the CAR-T cell therapy on Gupta.
Asked about the therapy’s success rate, he stressed that the initial findings suggest “better survival chances and lower remission rates” for patients in early stages of cancer. “We will need two years to determine the treatment’s success level. So far, all the patients who underwent this therapy at our hospital are in complete cancer remission with no cancer signs detectable through diagnostic tests. Years of data are needed to identify potential relapse timelines for patients, if any are ever reported among these patients,” he said, adding that this advanced gene therapy is expected to “reduce cancer-related mortality, revolutionise cancer treatment and save hundreds of lives”.
‘Feel like a soldier — tired, but unwilling to give up’
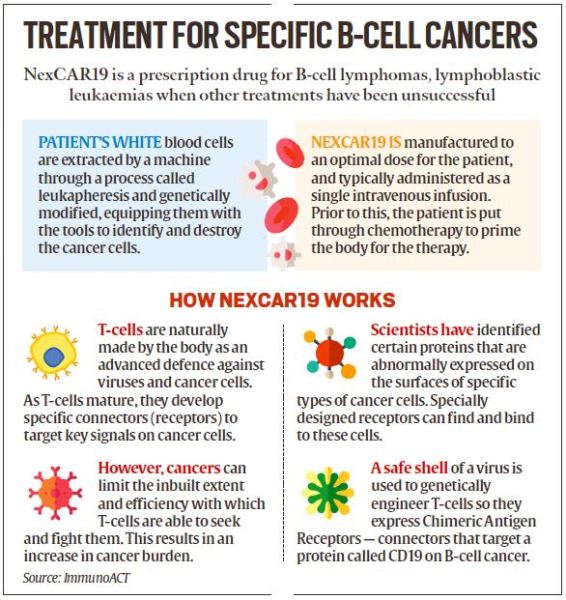
NexCAR19, the indigenously developed therapy that Gupta underwent, is a treatment for B-cell cancers (types of cancers that form in the immune system’s cells) such as leukaemia and lymphoma. It has been developed collaboratively by ImmunoACT, a company incubated at the Indian Institute of Technology Bombay (IITB), IIT-B and Tata Memorial Hospital.
The commercial use of this therapy was approved by the Central Drugs Standard Control Organisation (CDSCO) in October 2023. Currently, NexCAR19 is available in over 30 hospitals in more than 10 cities in India. Patients over the age of 15 years who suffer from B-cell cancers are eligible for this one-time therapy at these centres.
Gupta, who served as a doctor in the Army for 28 years, suffered from acute lymphoblastic leukaemia, a rapidly growing cancer that impacts white blood cells, specifically lymphocytes. “When my (bone marrow) transplant failed (in 2022)…as a doctor myself, I knew I only had a few months left to live. But the CAR-T cell therapy saved me. I feel like a soldier now — tired, but unwilling to give up,” he told The Indian Express.
As part of the therapy, the patient’s T-cells (types of immune cells) are collected and genetically modified into potent cancer fighters known as CAR-T cells so that they express chimeric antigen receptors (CARs) specific to cancer cells. The modified CAR-T cells are then expanded in the laboratory before being infused back into the patient. Once in the body, these engineered cells recognise and attack cancer cells, with a focus on B-cell cancers, thus offering a targeted and potent immunotherapy.
“Our therapy is tailored for patients with B-cell lymphomas, those in a relapsed or refractory state. These are individuals for whom traditional treatments like chemotherapy have failed, leading to a relapse,” said Dr Rahul Purwar, Associate Professor of Bioscience and Bioengineering at IIT-Bombay and founder-CEO of ImmunoACT.
This was the case with Gupta too. In 2021, he was diagnosed with cancer and underwent a four-week chemotherapy regimen without any improvement. A Canada-based doctor friend consulted a local haematologist, who designed a specialised treatment regimen for Gupta. The tailored regimen led to improvements in his health and a decline in cancer cells. In January 2022, his daughter donated her bone marrow but the transplant failed and his cancer returned.
Subsequently, his doctor friends recommended CAR-T cell therapy. However, inquiries in Singapore, the UK and Israel revealed that they cost over Rs 3 crore. Around that time, Gupta discovered the start of the commercial CAR-T cell therapy in India and enrolled for it at Mumbai’s Tata Memorial Hospital in November 2023.
“My blood was collected in mid-November and the T-cell transfusion took place on November 30. In just two weeks, my cancer cell count decreased significantly and my haemoglobin levels started rising,” Gupta told The Indian Express. Gupta’s treatment costs were covered under the Ex-Servicemen Contributory Health Scheme (ECHS) given his military service.
First CAR-T cell therapy to get CDSCO approval
Fifteen patients suffering from B-cell cancers, including Gupta, have accessed this treatment commercially. Even as results for the others are eagerly awaited, there are two crucial evaluation points when it comes to the final outcome.
“The first step takes place 28 days after the blood infusion with CAR-T cells with researchers doing a PET scan (an imaging test that uses a radioactive substance to look for disease),” said Shirish Arya, co-founder and director of Corporate Strategy and Business Development at ImmunoACT, adding that not all patients have reached the 28-day milestone.
“The second evaluation point takes place after three months. Both milestones are pivotal in determining the success of the treatment. The first three patients (including Gupta) are in complete remission, marking a significant positive outcome,” added Arya.
Gupta said, “My body has been declared free of cancer cells. I did not have any major side effects either, just some minor sinus-related issues. In fact, I rejoined my OPD (outpatient department) duties at Max Hospital (in Delhi) on February 2.”
While ImmunoACT’s is the first CAR-T cell therapy to get CDSCO approval, many hospitals, including Chandigarh-based Postgraduate Institute of Medical Education and Research (PGIMER), are also conducting groundbreaking clinical trials in the use of CAR-T cell therapy to treat cancer.
The commercial use of this therapy to treat certain blood cancers was approved by the Central Drugs Standard Control Organisation (CDSCO) in October 2023.
Pulitzer Prize-winning oncologist Siddhartha Mukherjea is also conducting his first phase II multicentre CAR-T cell therapy study. His start-up Immuneel is working on the therapy.







