DCGI
DCGI NEWS
Enforce global standards by January, launch inspections: DCGI to states
November 11, 2025 5:15 am
The big pharma companies, with a turnover of more than Rs 250 crore, were asked to implement the stricter norms within six months; smaller companies were given a year.
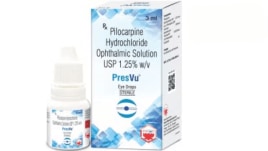
Drug regulator suspends pharma firm from manufacturing eye drops
September 11, 2024 8:03 pm
DCGI takes note of product's promotion on social media
India’s cervical cancer vaccine found to be safe and effective as Merck’s Gardasil vaccine: Why are the findings of the new Lancet study important?
November 08, 2023 10:43 pm
A new study reveals that HPV vaccine by the Serum Institute of India, based in Pune, elicits a comparable immune response to Merck's vaccine.
Drug regulator advises against using cough syrup with pholcodine. What is it about? Whom does it concern?
July 20, 2023 8:43 pm
The opiate pholcodine has been linked to severe allergic reactions in people who undergo surgeries with general anaesthesia later on. These have manifested as drop in blood pressure, loss of blood circulation, abnormal rhythm of the heart, closing up of airways and low oxygen levels
After WHO safety alert, DCGI issues advisory against pholcodine-containing cough syrups; all you need to know
July 20, 2023 4:00 pm
According to WHO, it can trigger life-threatening anaphylactic allergic reactions if people are also given specific muscle relaxant drugs while under a general anaesthetic
AstraZeneca Pharma India gets DCGI's nod for cancer-treating drug
May 03, 2023 9:54 pm
HER2 is a form of growth-promoting protein expressed on the surface of multiple tumours, including breast, gastric, lung and colorectal cancers, and is one of the biomarkers expressed in breast cancer tumours, it added.
Amazon, Flipkart among 20 e-tailors given notices for selling drugs without licence
February 12, 2023 7:27 pm
The show-cause notice dated February 8 by DCGI V G Somani cited a Delhi High Court order dated December 12, 2018, which prohibits online sales of medicines without a licence.
DCGI grants marketing authorisation to SII's qHPV vaccine against cervical cancer
July 12, 2022 8:18 pm
The government advisory panel NTAGI had recently also approved the qHPV after reviewing the clinical trial data of the vaccine.
Pune-based Gennova Biopharmaceuticals' mRNA vaccine gets DCGI nod
June 29, 2022 7:32 am
Gennova already has a license from the Central Drug Standard Control Organisation to manufacture and sell the vaccine and has produced 70 lakh doses at risk.
Serum Institute seeks EUA for its Covovax vaccine for 12-17 yrs age group
February 21, 2022 7:55 pm
The Drugs Controller General Of India has already approved Covovax for restricted use in emergency situations in adults on December 28.
Best of Express