Parag Gad’s company has developed two devices which help adults who lack bladder control due to a brain, spinal cord or nerve problem and children with cerebral palsy. Los Angeles-based Gad runs SpineX, a clinical-stage MedTech bioelectric company specialising in developing non-invasive tools for patients, which he started in 2019.
“Imagine a pacemaker, but instead of targeting your heart, this pacemaker activates the spinal cord. And rather than being an implantable device requiring surgery, it’s non-invasive. We place it directly over the back at various levels,” Gad, Co-founder and CEO, SpineX, explains.

A biomedical engineer, Gad graduated from a University in Mumbai in 2008 before moving to the US, where he pursued his master’s and then PhD from the University of California, Los Angeles, specialising in neuroengineering and rehabilitation. He first developed the technology at UCLA, where he was a full-time scientist for five years before starting SpineX. Gad has more than 15 years of preclinical and clinical research and product development experience.
SCONE and SCiP
 Neuromodulation therapies help to re-establish normal function of the nervous system. (Image credit: SpineX)
Neuromodulation therapies help to re-establish normal function of the nervous system. (Image credit: SpineX)
Gad’s SpineX has developed two products: Spinal Cord Neuromodulator (SCONE), for adults, which helps in the treatment of neurogenic bladder due to spinal cord injury, multiple sclerosis, and stroke; and Spinal Cord Innovation in Pediatrics (SCiP), a device that can be used in the treatment of movement disorders in cerebral palsy (CP) during childhood, affecting a person’s ability to move and maintain balance and posture.
Both devices are essentially based on the same technology, spinal cord stimulation, also known as neuromodulation, which involves placing electrodes next to the precise area of the spinal cord that is the source of pain. As Gad says, the only difference between the two is that the adult device transmits higher current whereas the pediatric device uses less current, keeping children in mind.
Gad says SCONE, the spinal cord neuromodulation device, is as small as the size of an old-school pager and battery-powered. It is connected via wires to the back, which deliver impulses that can stimulate specific structures in the spine and result in the release of various inhibitory neurotransmitters that, in turn, block neural impulses that cause pain.
 Gad’s company has been testing the technology for the past few years, with clinical trials for SCONE running around the world, including two sites in India. (Image credit: SpineX)
Gad’s company has been testing the technology for the past few years, with clinical trials for SCONE running around the world, including two sites in India. (Image credit: SpineX)
“Walking is an automatic process,” he explains. “When there is trauma that occurs, the communication or the neurons in the brain and spinal cord are now miscommunicating. With the electrical treatment, we rewire how the brain and spinal cord communicate with the rest of the body. That’s really the core of the therapy here: by delivering these impulses into the spine, you’re correcting the communication that’s occurring in the brain, spinal cord, and muscles.”
Story continues below this ad
Traditionally, physical therapy is recommended to address movement problems associated with cerebral palsy among children. However, Gad argues that physical therapy can only manage pain but doesn’t lead to any functional improvement.
“The magnitude of change that’s possible for a child who is unable to move his legs, to now be able to ride his bike at home, and the fact that you’re able to cause a physical change in the nervous system so that they can retain this improved function at home, even when the treatment is not actively being delivered—that is really the beauty of this treatment,” he said. “We are the first of its kind,” he continues.
A home-use device
 SciP is aimed at children with cerebral palsy. Gad expects the SciP device to get approval in India in 2026. (Image credit: SpineX)
SciP is aimed at children with cerebral palsy. Gad expects the SciP device to get approval in India in 2026. (Image credit: SpineX)
SCONE works in conjunction with a companion app that allows therapists, users, caregivers, and physicians to monitor the efficiency of the therapy. It has an AI interface that enables users to obtain more optimised stimulation parameters and provides prompts regarding when to perform stimulation therapy. “If the treatment is done one hour a day or twice a week for eight weeks, how do you know which two hours to stimulate and which two hours you should train? With that AI interface, it will accumulate a ton of data, and based on what we have seen over the last five days, we would have the best outcome for the next three days,” Gad explains.
It is noninvasive, so it is safe and doesn’t require multiple procedures, and more importantly, frequent visits to a clinic. “It’s potentially a home-use device. You’re not expected to come to a clinic three or four times a week. Patients come in initially to get assessed, receive the device as per their requirements, and take it home with them to continue therapy at home,” says Gad.
Story continues below this ad
Moreover, Gad says a patient is free to figure out if the neuromodulation therapy works, so they don’t have to invest six months, thus saving a lot of time. He recommends that patients take a one- or two-day session and then enrol for longer sessions. “This is classified as what’s called a non-significant risk device. It has a very low-risk profile and has almost no adverse events on the patient population,” claims Gad.
Gad clarifies the device will only be made available to patients upon a doctor’s recommendation of going for a non-pharmacological option instead of a medication. “It’s a medical device and not an over-the-counter (OTC) medical device that may be offered for sale directly to the consumer, meaning it requires a prescription. It is not a one-size-fits-all; it has to be customised for the patient,” he says. “That’s where the physician comes into the picture. They will acquire the device from us, but then it will be set based on the patient’s needs by a qualified healthcare professional.”
‘Breakthrough products’
 Gad says about 85% of the patients don’t continue drugs because of all the side effects that they have to face. (Image credit: SpineX)
Gad says about 85% of the patients don’t continue drugs because of all the side effects that they have to face. (Image credit: SpineX)
Right now, Gad’s SpineX is working with the US Food and Drug Administration (FDA) and the Central Drugs Standard Control Organisation (CDSCO) in India for simultaneous approval for the devices in both countries.
Gad’s company has been testing the technology for the past few years, with clinical trials for SCONE running around the world, including two sites in India: the Institute of Brain & Spine and Sri Balaji Action Medical Institute. It has also begun operations in Bengaluru in collaboration with WalkAgain, which runs rehabilitation programmes for people with neurological disorders.
Story continues below this ad
As for SCiP, Gad expects a multicenter pivotal trial to commence in H1 2024. Both of SpineX’s products have been designated by the FDA as “breakthrough products.”
For now, Gad is working on scaling the business model and reaching out to more physicians who recommend SpineX’s products based on the unique bioelectric platform technology. “When you talk about scalability, the fact that you can translate this to home use and allow people to do it by themselves is great in making sure that this is a scalable model.”
“Our goal is to get this therapy to the patients. So whether it is a clinical device used when they come into the clinic for therapy, or if it is a home-based device that the patient takes home, we make sure that the patient is receiving the therapy,” he says.
Gad is closely studying how to price the device in India. However, the final pricing also depends on how much a hospital is willing to pay. He says the price would be different for a hospital device and a home device. Gad is targeting the home device to be priced “cheaper than a cell phone.”



 Neuromodulation therapies help to re-establish normal function of the nervous system. (Image credit: SpineX)
Neuromodulation therapies help to re-establish normal function of the nervous system. (Image credit: SpineX)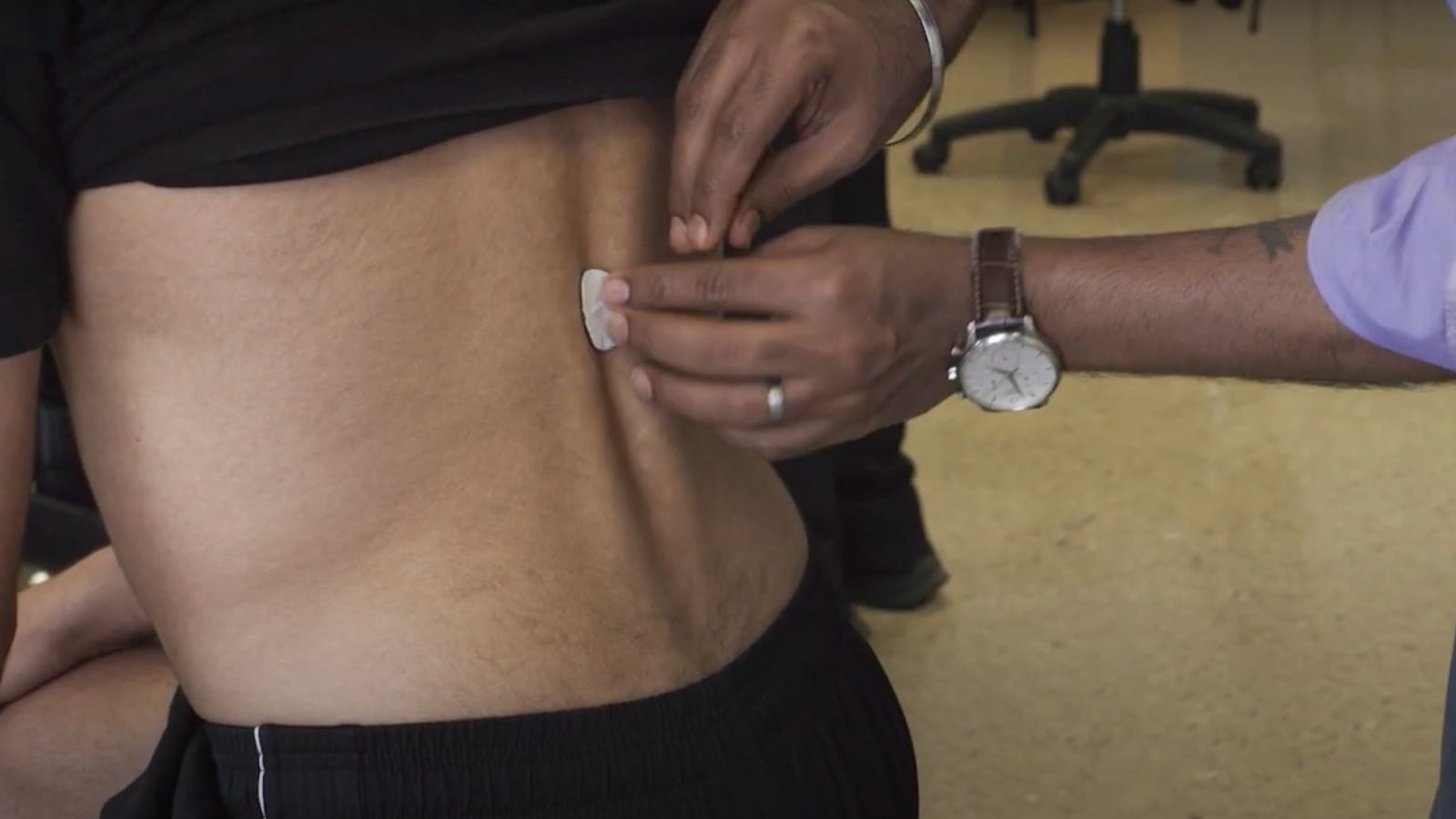 Gad’s company has been testing the technology for the past few years, with clinical trials for SCONE running around the world, including two sites in India. (Image credit: SpineX)
Gad’s company has been testing the technology for the past few years, with clinical trials for SCONE running around the world, including two sites in India. (Image credit: SpineX) SciP is aimed at children with cerebral palsy. Gad expects the SciP device to get approval in India in 2026. (Image credit: SpineX)
SciP is aimed at children with cerebral palsy. Gad expects the SciP device to get approval in India in 2026. (Image credit: SpineX) Gad says about 85% of the patients don’t continue drugs because of all the side effects that they have to face. (Image credit: SpineX)
Gad says about 85% of the patients don’t continue drugs because of all the side effects that they have to face. (Image credit: SpineX)





