24 crore people vaccinated so far, Govt says over 25.87 crore doses provided to states
Dr V K Paul, who heads the country’s Covid task force, said the US decision will not have any regulatory impact in India.
monthly limit of free stories.
with an Express account.
 1 / 10
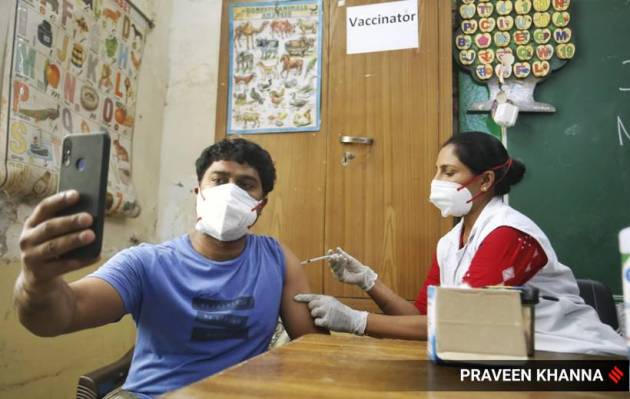
1 / 10As of Saturday, vaccine doses have been administered to as many as 24,96,00,304 people in India under the nationwide vaccination drive.
- 2 / 10
More than 25.87 crore COVID-19 vaccine doses have been provided to states and union territories so far through the Centre and direct state procurement category, the Union health ministry said on Saturday. Furthermore, the ministry said more than 10,81,300 vaccine doses are in the pipeline and will be received by states and union territories within the next three days.
- 3 / 10
Meanwhile, publication of crucial phase 3 trial data of Bharat Biotech’s Covaxin, which will detail the efficacy of the indigenous Covid vaccine developed by ICMR and Bharat Biotech, will done in the next “7-8 days”, according to Dr V K Paul, who heads the country’s Covid task force.
- 4 / 10
Dr Paul said the data will be published in a peer-reviewed journal, “go beyond” information submitted so far to the Central Drugs Standard Control Organisation (CDSCO), and reveal findings from “follow-ups” of the clinical trial.
- 5 / 10
The announcement came on a day the US FDA recommended that Covaxin makers apply for full authorisation instead of seeking Emergency Use Authorisation (EUA), thereby delaying a rollout of the vaccine in that country.
- 6 / 10
Ocugen, Bharat Biotech’s Covaxin partner in the US, “will no longer pursue an Emergency Use Authorization” of the vaccine in that country, the American clinical stage biopharmaceutical company told Nasdaq on Thursday.
- 7 / 10
However, the decision to not consider Covaxin for Emergency Use Authorisation (EUA) in the US is unlikely to have any bearing on the WHO’s ongoing review of the Indian Covid vaccine for Emergency Use Listing (EUL), according to experts. It will also have “no bearing” on India’s own vaccination programme.
- 8 / 10
The US Food and Drug Administration (FDA), in its feedback regarding Ocugen’s “master file” for Covaxin, had recommended that the company pursue a Biologics Licence Application (BLA) “instead of an EUA application”.
- 9 / 10
Paul also said the US decision will not have any regulatory impact in India. According to the company, regulatory approvals from WHO are expected by July-September 2021. Currently, Covaxin comprises approximately 12 per cent of total vaccines administered in India.
- 10 / 10
On Covaxin’s phase 3 data, he said: “We have so much data on their safety; we have so much data on their phase 3 trial that has been screened by them, seen by our regulator. I am told that their phase 3 publication could come anytime in the next 7-8 days. This will be beyond what has been shared with the DCGI (Drugs Controller General of India), when they received the licensure; the follow-up (data) will go into a peer-reviewed journal. It will be done objectively.”