Anonna Dutt is a Principal Correspondent who writes primarily on health at the Indian Express. She reports on myriad topics ranging from the growing burden of non-communicable diseases such as diabetes and hypertension to the problems with pervasive infectious conditions. She reported on the government’s management of the Covid-19 pandemic and closely followed the vaccination programme. Her stories have resulted in the city government investing in high-end tests for the poor and acknowledging errors in their official reports. Dutt also takes a keen interest in the country’s space programme and has written on key missions like Chandrayaan 2 and 3, Aditya L1, and Gaganyaan. She was among the first batch of eleven media fellows with RBM Partnership to End Malaria. She was also selected to participate in the short-term programme on early childhood reporting at Columbia University’s Dart Centre. Dutt has a Bachelor’s Degree from the Symbiosis Institute of Media and Communication, Pune and a PG Diploma from the Asian College of Journalism, Chennai. She started her reporting career with the Hindustan Times. When not at work, she tries to appease the Duolingo owl with her French skills and sometimes takes to the dance floor. ... Read More
‘From Rs 18 lakh to Rs 48,000 a month, I can finally afford therapy within my salary’: Delhi HC allows Natco to manufacture Roche’s rare disease drug
Natco Pharmaceuticals wins case against international pharmaceutical giant Roche; patients of spinal muscular atrophy heave a sigh of relief.
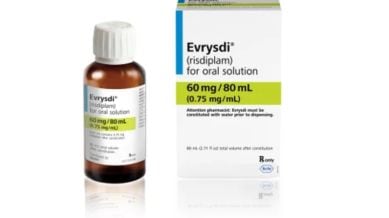
Lucknow’s Saifullah Khalidi and Delhi’s Purva Mittal breathed a sigh of relief when the Delhi High Court on Thursday decided not to stop Natco Pharmaceuticals from manufacturing a generic version of international pharmaceutical giant Roche’s Risdiplam. The medicine can help patients with spinal muscular atrophy — a rare disease that progressively affects muscle functions — in retaining their motor functions. Although both are in their early 30s, the disease has left both of them dependent on others for their day-to-day functioning.
The refusal of an injunction against Natco Pharmaceutical means Khalidi and Mittal will be able to start taking a cheaper, affordable version of the drug. With each bottle of the branded drug, Evrysdi, costing around Rs 6 lakh — and most adults needing around three a month — the total cost of R 18 lakh was unimaginable for them. Natco Pharma released a statement, saying, “The company has decided to launch the product with immediate effect and price the product at Rs 15,900 MRP consistent with the company’s stand before the court.” With this, the annual cost for the treatment will be around Rs 5-6 lakh.
monthly limit of free stories.
with an Express account.
Why any relief works for continued treatment
Although not entirely affordable, this is something that families can manage. “Even at this price point, it will cost me around half my salary. But, at least, I can afford it,” said the 31-year-old assistant professor from Delhi University. Natco Pharmaceutical also “intends to offer some discount to certain deserving patients through its patient access programme.”
While it is more difficult for Saifullah, whose father has retired as an engineer and who doesn’t make a lot as a freelance content writer, he hopes to avail some assistance programmes. The reduced cost may mean that patients would be able to access the treatment for longer under the government’s Rare Disease Policy, which allows for a one-time Rs 50 lakh fund per patient for treatment. While 26-year-old Seba PA used the fund for the branded drug — which helped her prevent hospitalisation due to recurrent chest infections — her funding dried up within nine months. With the reduced cost of the generic drug, other patients may be able to use the medicine for around nine years.
The patent battle
The decision is significant considering that it grants the patients and activists significant grounds for challenging Roche’s patent. Explained an expert on patent laws, “The initial patent that was filed by the company in the US and other countries was never filed in India. The Indian patent for the molecule was filed later, which Natco claims does not stand because the drug could not be considered novel with the formulation already having been disclosed in the previous patent.”
Roche claimed that the first genus patent — a patent for a family of substances and not a single formulation — did not specifically disclose the structure of Risdiplam. The later species patent — a patent for a specific product from the family — is what contains the exact structure. Natco pharmaceutical countered this, saying that there was only one substitution needed in the already disclosed formula, which anyone with the knowledge in the field can arrive at given the information disclosed in the first patent. The single judge and the division bench agreed that Risdiplam’s patent was “vulnerable to invalidity” and, therefore, did not grant an injunction to stop Natco Pharma from manufacturing the medicine.
This is significant considering that a compulsory licence has been granted only once in the last 20 years since the country’s new Patent Act came into effect. Natco Pharma in 2012 had secured the permit to manufacture Nexavar, a cancer drug made by German multinational Bayer. Since then, three Indian companies have unsuccessfully applied for compulsory licenses — BDR Pharmaceuticals for Dasatinib (for leukemia), Lee Pharma for Sitagliptin (for diabetes), and Emcure Pharmaceuticals for Trastuzumab (for breast cancer)
Patients with SMA had previously filed petitions urging the government to invoke compulsory licensing — a provision in the Patent Act that allows a non-patent holding company to manufacture a drug without consent of the patent holder.
Roche issued a statement, saying, “We are considering our options within the scope of the Indian law.”
Misinformation and doubts
While some eagerly await the generic version of the drug to enter the market, several SMA patients said that seeds of doubts and dissemination of disinformation had already started. “Some patients were told that there was no data to show that the generic version was effective, there were no trials, there were no approvals. With the price still being significant, many may hesitate. But if the company has gone after Natco, it knows that the product works,” said one of the patients.